The eTimestrip TIR temperature recorders continuously monitor temperature and calculating remaining stability budget of pharmaceutical products or clinical studies along the entire supply chain.
They are thin and small enough to go on clinical kits and sales units and durable enough to stay on the pharmaceuticals throughout the monitoring time.
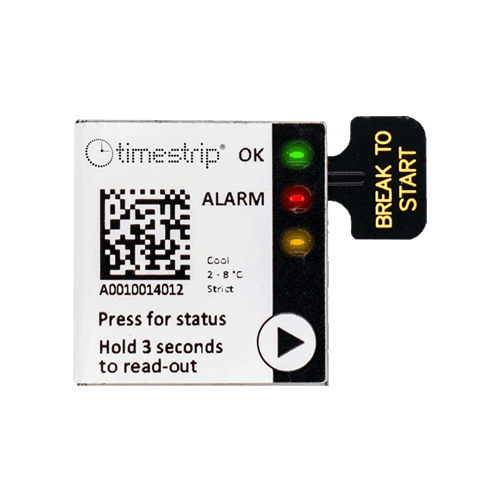
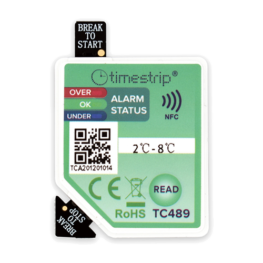
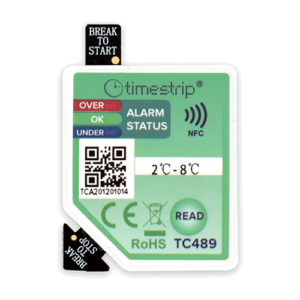
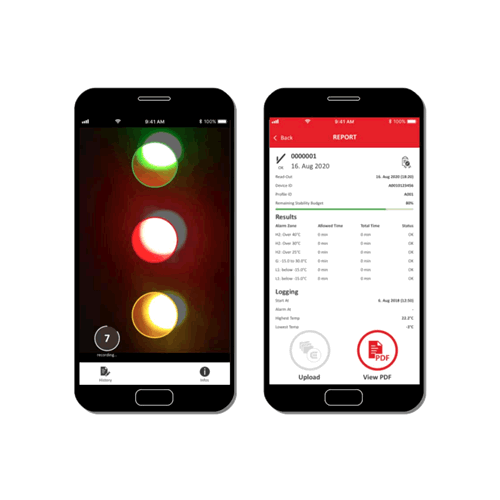
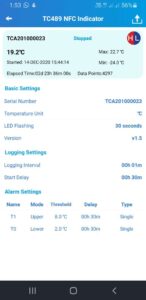
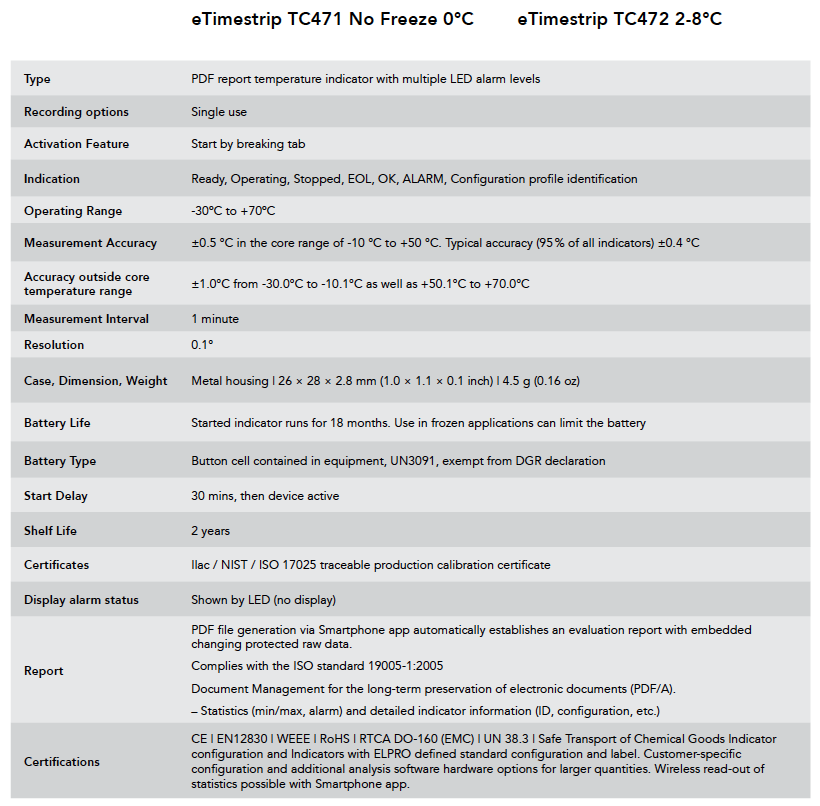
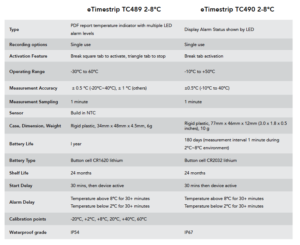
There are four eTimestrip TIR devices available. Use TC471 Freeze 0ºC Descending and TC472 2º-8ºC to manage deviations up to 6 alarm zones. The alarm status is always visible and can be extracted via compatible Android app. It is durable enough to stay on the product during its entire shelf life of up to 2 years. Use TC489 for 2º-8ºC shipments where a visual alarm status along with a downloadable PDF or Excel report via compatible Android app is required. Use TC490 for or 2º-8ºC shipments where a visual alarm status only is required.
The electronic indicators monitor temperature exposure and not the product quality. Its purpose is to indicate if product quality evaluation/ testing is required.
Complies with the ISO standard 19005-1 Document Management for the long-term preservation of electronic documents (PDF/A).
Activation tab
Timestrip TIR comes preconfigured as either a 0°C (do not freeze) or 2°C-8°C recorders. Once your product is ready to be shipped, tear off the tab to activate. Temperature monitoring will then begin after a start delay period of 30 mins.
Long Shelf Life
Durable enough to stay on the product during the entire shelf life of 2 years.
Features
| Thin, small, durable, smart and cost-effective | |
| Quick implementation | |
| Can be used by 3rd parties | |
| 100% calibrated and observes conformity to regulatory guidelines | |
| Stable monitoring along the entire supply chain | |
| Fully FDA GxP / 21 CFR Part 11 compliant | |
| LED indication, PDF download and mobile app versions |